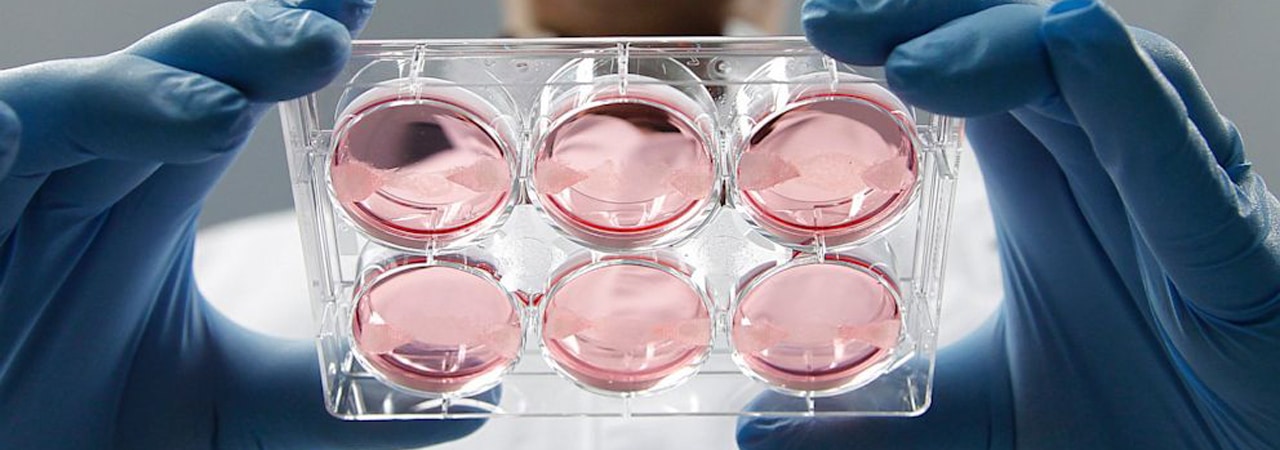
In 1931, Wintston Churchill speculated on the future of laboratory-grown meat in his essay Fifty Years Hence. He wrote: “We shall escape the absurdity of growing a whole chicken in order to eat the breast or wing by growing these parts separately under a suitable medium". Even before animal welfare conditions and environmental damage, Churchill foresaw the benefits of simple animal tissues over raising animals as a whole.
The Food and Drug Administration (FDA) first approved in-vitro techniques for commercial meat production in 1995, followed by the first received patent in 1999. In 2008 PETA publicly supported the in-vitro cause and announced a one million dollar prize for the first commercially viable lab-grown chicken. Even though the deadline for the prize expired in March 2014, the lab work on cultured meat has come a long way.
However, “No one is currently funding this kind of research” says Isha Datar, CEO of New Harvest, a non-profit organization that addresses all-encompassing food insecurity. “Mainly because it is at the intersection of food and medical science” she adds. Until date, there has been a clear division between the US Department of Agriculture (USDA) and the FDA, but as emerging biotechnologies are blurring the lines of oversight, it is important to rethink these regulatory definitions.
A common strategy used by industry leaders is to show similarities between lab-grown products and existing ones. According to Datar “Most food regulation is about aligning new products with something that’s already recognized as safe”. Nicole Negowetti, policy director at Good Food Institute, comments, “It’s uncharted territory […] from my understanding, the USDA regulations are based on food from animal slaughter, so [they don’t] make sense for these products”.
In 2015 the White House first launched a panel to clarify the roles of the agencies that regulate agricultural biotechnology. In addition, last June the National Academies of Sciences, Engineering and Medicine, in Washington DC, held a public meeting on biotech, with a report scheduled later this year. According to Datar, “Our system is set up in a way that promotes imitation as opposed to innovation” therefore she suggests establishing a single regulatory agency would be more efficient.
Read more at Science Magazine. Image: Reuters
Share your thoughts and join the technology debate!
Be the first to comment